Europe’s New Health Technology Assessment Framework: Progress or Pitfall?

On January 12, 2025, the European Union’s Health Technology Assessment Regulation (HTAR) came into force, introducing a centralized framework for the evaluation of medicines and medical devices across Member States. At first glance, the regulation promises to streamline processes, eliminate duplication, and expedite patient access to innovative treatments. However, beneath this ambitious exterior lies a system that faces considerable logistical and operational challenges.
A Framework of Ambition
The HTAR mandates joint clinical assessments (JCAs) for certain medicines and high-risk medical devices, with a focus on evaluating their clinical benefits relative to existing treatments. By pooling resources at the EU level, the regulation aims to reduce the burden on developers, who will now submit a single dossier for evaluation. It also imposes strict timelines, requiring assessments to be completed within 30 days of a medicine’s marketing authorization.
Yet, the regulation stops short of full harmonization. Member States retain the authority to conduct their own non-clinical assessments—considering economic, social, and ethical factors—and to decide on the adoption and reimbursement of health technologies. This dual-layered approach risks undermining the efficiency gains that a centralized system ostensibly offers.
The Challenge of Implementation
While the HTAR aspires to address inefficiencies in the fragmented HTA landscape, its practical execution raises questions. The European Medicines Agency (EMA) has been tasked with supporting the HTA Coordination Group (HTACG), which oversees JCAs. However, EMA’s capacity to manage this additional workload is unclear.
In 2023, EMA recommended 77 medicines for marketing authorization, a pace that far exceeds the HTACG’s anticipated ability to assess just 25 drugs in 2025, including 17 cancer treatments and 8 advanced therapy medicinal products (ATMPs). This gap suggests that the system may struggle to meet demand, particularly as the scope of the HTAR expands to orphan medicines in 2028 and all centrally authorized drugs by 2030.
National Considerations Persist
Despite the promise of reduced duplication, national authorities will continue to play a significant role in decision-making. While JCAs aim to provide a unified clinical assessment, Member States remain free to draw their own conclusions on relative effectiveness and to impose additional requirements for non-clinical assessments. For developers, this means navigating a complex web of national priorities, potentially negating some of the procedural efficiencies the HTAR seeks to achieve.
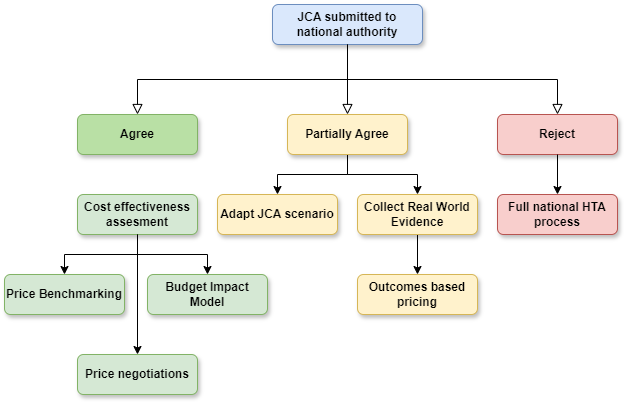
Stakeholder Involvement and Bottlenecks
The regulation introduces mechanisms for involving patients, clinicians, and other stakeholders in the assessment process. While this inclusive approach is commendable, it also adds layers of complexity. Consultation processes, combined with tight timelines, could create bottlenecks, further straining the system’s already limited capacity.
EMA’s role in horizon scanning and parallel joint scientific consultations (JSCs) adds another layer of complexity. In 2025, the HTACG plans to initiate just 5 to 7 JSCs for medicinal products, a modest figure given the scale of the EU healthcare market. Whether this limited capacity can deliver meaningful support to developers remains an open question.
An Uncertain Road Ahead
The HTAR represents a bold attempt to harmonize HTA processes across the EU, but its success is far from guaranteed. The regulation places significant demands on EMA and national authorities, whose capacity to handle the workload is yet to be proven. Moreover, the continued dominance of national considerations risks perpetuating inefficiencies, undermining the benefits of centralization.
As the HTAR takes effect, the EU faces a delicate balancing act. The promise of faster access to innovative therapies for patients must be weighed against the practical realities of implementation. For now, the HTAR offers more questions than answers. Its success—or failure—will depend on the EU’s ability to translate ambition into action without collapsing under the weight of its own complexity.
Member discussion